Vitamin stability
The stability of vitamins in a premix is critical in maintaining vitamin potency. Susceptibility to degradation varies depending on individual vitamins and on a number of factors that affect vitamin stability. Safety margins for vitamin premix formulation are usually based upon vitamin cost, presence or absence of trace minerals and choline in the premix, feed processing characteristics, environmental conditions, anticipated storage time, and expected rates of vitamin potency losses (Shurson et al., 2011).
Factors affecting vitamin stability
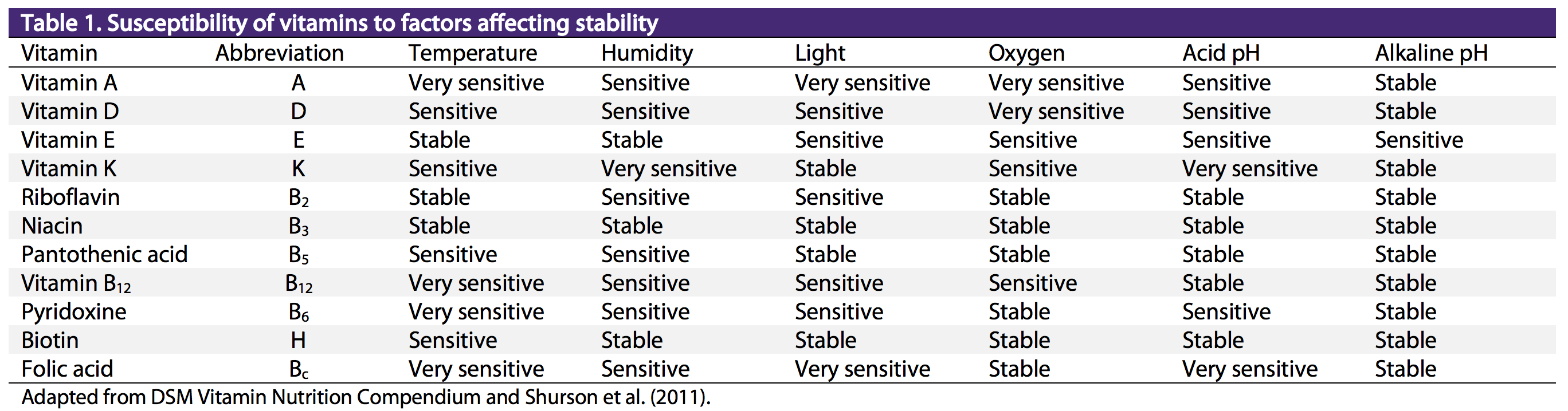
Vitamin stability in premixes is affected by exposure to light, heat, moisture, oxygen, and pH, and contact with other compounds. These factors subject vitamins to degradation primarily through oxidation. The long-term or multiple exposure to these factors generally magnifies the negative impact on vitamin stability. The individual vitamins vary in their susceptibility to degradation ( Table 1 ). In general, the most sensitive or labile vitamins are vitamin K3, vitamin A, pyridoxine, vitamin B12, and folic acid (Shurson et al., 2011).
Vitamin stability in premixes
Premix composition affects vitamin stability, especially with regard to the presence or absence of choline and inorganic trace minerals. To maintain vitamin potency it is recommended to have vitamin premixes separated from choline and trace mineral premixes.
Choline is very hygroscopic and absorbs significant amounts of moisture from the environment, which affects the stability of other vitamins when added in the premix. Inorganic trace minerals also affect the stability of vitamins when added in the premix, as trace minerals often produce reduction and oxidation reactions in the premix. Among the inorganic trace mineral sources, the sulfates have greater effect on vitamin stability than carbonates and oxides. Use of organic trace mineral sources reduces vitamin activity losses by 40 to 50% during storage compared to adding inorganic trace minerals in a vitamin-trace mineral premix (Shurson et al., 2011).
Vitamin stability during feed processing
Some processes used in feed manufacturing affect vitamin stability (Reddy and Love, 1999). In swine diets, pelleting is typically the most aggressive process against vitamins due to exposure to heat, moisture, pressure, and abrasion. Mixing also affects vitamin stability due to abrasion and contact with other compounds in the diet.
Vitamin stability during storage
Vitamins are rather stable prior to preparation of a premix and remain reasonably stable in complete feeds. Consequently, most vitamin losses occur while the vitamin premix is under storage. Both storage time and storage conditions should be controlled for vitamin premixes. Vitamin premixes should be stored in a dry, cool, and dark place to maintain stability during storage. Also, the use of barriers such as plastic-lined bags aid in reducing the absorption of moisture.
Premixes containing vitamins exclusively can be stored for about 3 to 4 months. However, storage time should not exceed 60 days if choline and trace minerals are present in combination with vitamins in the premix.
During storage, vitamin potency can be monitored in premixes through a vitamin activity assay. Vitamin assay costs are generally expensive, which prompts the selection of one indicator vitamin to estimate the vitamin potency losses in the premix. According to Shurson et al. (2011), retinol appears to be the best indicator in the vitamin premix because of the low cost of assay, relatively high sensitivity of vitamin A to multiple factors, and high expected loss of activity per month of storage.
Technologies to improve vitamin stability
Advances in research and technology have led to the development of specialized vitamin forms to provide superior vitamin stability. Many commercial vitamin manufacturers have succeeded in enhancing stability of vitamins with spray-drying and beadlet technologies (DSM Vitamin Nutrition Compendium).
Spray-dried vitamin products are manufactured as a fine powder with high stability and good uniformity of mix, but only fair quality in terms of flowability, dustiness, and caking. Beadlets are produced by coating vitamins in gelatin or starch to prevent contact with factors affecting vitamin stability until it is digested by the pig. Beadlets are manufactured as a fine granular product with high stability and also high flowability, low dustiness, and low caking characteristics. Cross-linked beadlets are produced by coating vitamins with cross-linked gelatin proteins, which makes harder beadlets that are more resistant to the pressure and abrasion of pelleting. The inclusion of antioxidants in the beadlets provide additional protection against oxidative factors.